Navigation Menu 
[expand all...]
[COLLAPSE ALL...]

|
Polymer Mixing

 Table:
Table:
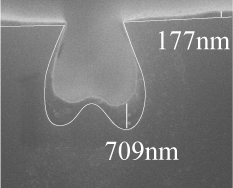
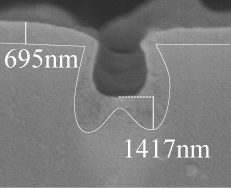
| Fiber Cross-Section |
PMMA |
DR1 azo dye |
Methy Ethyl Ketone |
Clorobenzene |
 |
8g |
0.6g |
37.5ml |
112.5ml |
 |
8g |
0.6g |
30ml |
90ml |
 |
8g |
0.6g |
15ml |
45ml |
Expand All
Compress All
- Materials needed:
- *For each batch*
- • (2) 8 oz glass wide-lid jars (from Chem-stores)
- • PMMA
- • DR1 - 95% or higher purity
- • MEK
- • Chlorobenzene
-or Cyclohexanone as solvent-
- • mass scale (to measure out weight in grams)
- • graduated cylinder
- • (1) 1 in. magnetic stir bar (from Chem-stores)
- • Electric Stirrer or Hotplate/stirrer (for cyclohexanone)
- • (1) Large disposable 60 mL syringe (from Chem-stores)
- • (1) .2 μm filter disposable (from Chem-stores)
PMMA with Chlorobenzene and MEK
- (Using more solvent in these recipes will yield thinner polymers)
- 1) Clean a glass jar using acetone and isopropanol. Dry it with the nitrogen gun.
- 2) Using a clean spoon and a plastic cup, measure 8g of PMMA on scale and put into glass jar
- 3) Using a clean spoon and plastic cup, measure 0.6g of purest DR1 available (95% or better) and put into glass jar
- 4) Measure 15 mL, 30 mL, or 37.5 mL of MEK in a clean graduated cylinder and pour into glass jar (for 1X, 2X, or 2.5X solvent recipes)
- 5) Measure 45mL, 90mL, or 112.5 mL of Chlorobenzene in a clean graduated cylinder and pour into the jar (for 1X, 2X, or 2.5X solvent recipes)
- 6) Place a clean stir bar into the jar
- 7) Put lid on the jar and tighten
- 8) Place the jar on the stirrer set at 6-7
- 9) Stir for 24 hours
- 10) Put a 0.2 micron filter on a 60 mL syringe
- 11) Draw the polymer through the filter into the syringe
- 12) Remove the filter and squirt the polymer into a clean container
PMMA with Cyclohexenone
- (Using more solvent in these recipes will yield thinner polymers)
- 1) Clean a glass jar using acetone and isopropanol. Dry it with the nitrogen gun.
- 2) Using a clean spoon and a plastic cup, measure 8g of PMMA on scale and put into glass jar
- 3) Using a clean spoon and plastic cup, measure 0.6g of purest DR1 available (95% or better) and put into glass jar
- 4) Measure 60 mL, 120 mL, or 150 mL of Cyclohexenone and pour into glass jar
- 5) Place a clean stir bar into the jar
- 6) Put lid on the jar and tighten
- 7) Place the jar on a heated stirring plate
- 8) Adjust the temperature to 44 degrees and monitor with a thermocouple
- 9) Let stir for 24 hours and ensure that temperature stays close to 44 degrees centigrade
- 10) Put a 0.2 micron filter on a 60 mL syringe
- 11) Draw the polymer through the filter into the syringe
- 12) Remove the filter and squirt the polymer into a clean container
How this works:
- • We use these polymer mixes in making our Core-replaced fiber sensors.
- • These mixtures have a guest-host relation between the polymer (PMMA) and the chromophore, or dye (DR1). The polymer gives the structure to the material, and the chromophore gives the material non-linear properties- which are extremely useful in optics and especially this application.
- • The solvents used (MEK & chlorobenzene, or cyclohexanone) allow the polymer and the chromophore to distribute evenly. When the material is allows to set or cure, the solvent evaporates leaving a polymer "matrix" with chromophore interdispersed all around. Then we "pole" the material.
- • The nonlinear properties of the chromophore (DR1) allow all the chromophore molecules to be aligned in a certain direction. This is done by "poling". The material is put in a large magnetic field under high temperatures which allows the material to soften and the chromophore to rotate.
- • The chromophores align themselves to the direction of the E-field because each molecule is a dipole.

|




